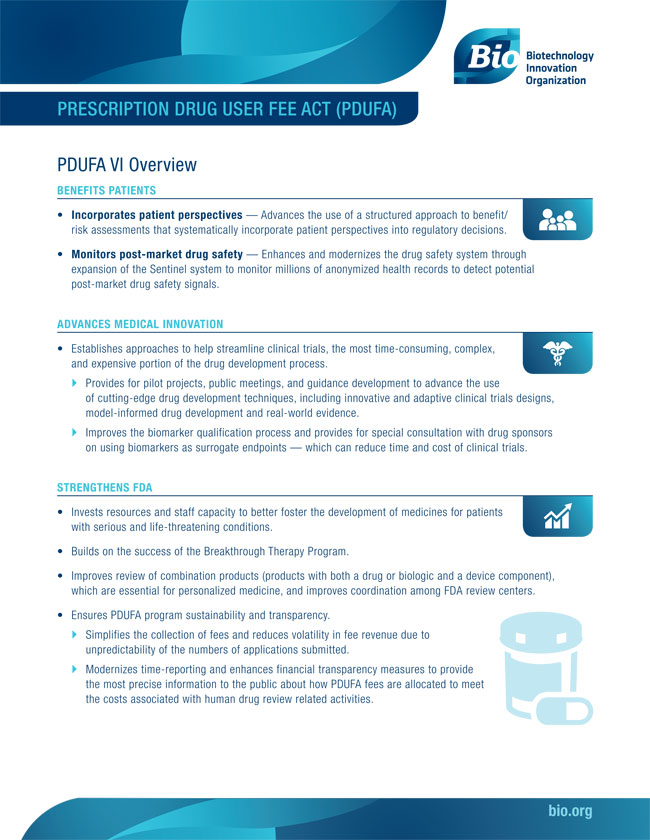
Meaning Of Pdufa . the prescription drug user fee act (pdufa) was created by congress in 1992 and authorizes fda to collect fees from. the final fda decision to approve or not approve a new product occurs on the prescription drug user fee act (pdufa) meeting date. the prescription drug user fee act (pdufa) was created by congress in 1992 and authorizes fda to collect fees from. In some instances, the fda grants priority review status to the regulatory filing for a drug. prescription drug user fee act (pdufa) pdufa was created in 1992 and authorizes the fda to collect fees from. the prescription drug user fee act (pdufa) was introduced to address the growing concern over the fda’s ability. the date at the end of the review period is referred to as the pdufa date.
from archive.bio.org
the prescription drug user fee act (pdufa) was introduced to address the growing concern over the fda’s ability. prescription drug user fee act (pdufa) pdufa was created in 1992 and authorizes the fda to collect fees from. In some instances, the fda grants priority review status to the regulatory filing for a drug. the final fda decision to approve or not approve a new product occurs on the prescription drug user fee act (pdufa) meeting date. the date at the end of the review period is referred to as the pdufa date. the prescription drug user fee act (pdufa) was created by congress in 1992 and authorizes fda to collect fees from. the prescription drug user fee act (pdufa) was created by congress in 1992 and authorizes fda to collect fees from.
PDUFA AtAGlance BIO
Meaning Of Pdufa the prescription drug user fee act (pdufa) was created by congress in 1992 and authorizes fda to collect fees from. the prescription drug user fee act (pdufa) was created by congress in 1992 and authorizes fda to collect fees from. the prescription drug user fee act (pdufa) was created by congress in 1992 and authorizes fda to collect fees from. In some instances, the fda grants priority review status to the regulatory filing for a drug. the prescription drug user fee act (pdufa) was introduced to address the growing concern over the fda’s ability. the final fda decision to approve or not approve a new product occurs on the prescription drug user fee act (pdufa) meeting date. the date at the end of the review period is referred to as the pdufa date. prescription drug user fee act (pdufa) pdufa was created in 1992 and authorizes the fda to collect fees from.
From ja.oliverhcp.com
PDUFAの影響力 Meaning Of Pdufa prescription drug user fee act (pdufa) pdufa was created in 1992 and authorizes the fda to collect fees from. the prescription drug user fee act (pdufa) was introduced to address the growing concern over the fda’s ability. the prescription drug user fee act (pdufa) was created by congress in 1992 and authorizes fda to collect fees from.. Meaning Of Pdufa.
From www.pdfprof.com
etc meaning Meaning Of Pdufa the final fda decision to approve or not approve a new product occurs on the prescription drug user fee act (pdufa) meeting date. the date at the end of the review period is referred to as the pdufa date. the prescription drug user fee act (pdufa) was created by congress in 1992 and authorizes fda to collect. Meaning Of Pdufa.
From www.offers.bpiq.com
Free PDUFA Analysis Report BiopharmIQ Meaning Of Pdufa the prescription drug user fee act (pdufa) was created by congress in 1992 and authorizes fda to collect fees from. prescription drug user fee act (pdufa) pdufa was created in 1992 and authorizes the fda to collect fees from. the date at the end of the review period is referred to as the pdufa date. the. Meaning Of Pdufa.
From www.slideserve.com
PPT Catalysts for enactment of PDUFA PowerPoint Presentation, free Meaning Of Pdufa the final fda decision to approve or not approve a new product occurs on the prescription drug user fee act (pdufa) meeting date. prescription drug user fee act (pdufa) pdufa was created in 1992 and authorizes the fda to collect fees from. the prescription drug user fee act (pdufa) was created by congress in 1992 and authorizes. Meaning Of Pdufa.
From www.slideserve.com
PPT PDUFA & FDA Legislation PowerPoint Presentation, free download Meaning Of Pdufa the final fda decision to approve or not approve a new product occurs on the prescription drug user fee act (pdufa) meeting date. the date at the end of the review period is referred to as the pdufa date. the prescription drug user fee act (pdufa) was created by congress in 1992 and authorizes fda to collect. Meaning Of Pdufa.
From www.researchgate.net
(PDF) PDUFA VI It's Time to Unleash the Full Potential of Model Meaning Of Pdufa prescription drug user fee act (pdufa) pdufa was created in 1992 and authorizes the fda to collect fees from. In some instances, the fda grants priority review status to the regulatory filing for a drug. the prescription drug user fee act (pdufa) was created by congress in 1992 and authorizes fda to collect fees from. the prescription. Meaning Of Pdufa.
From phrma.org
PDUFA VI Advancing a New Era of Medicine For Patients PhRMA Meaning Of Pdufa the prescription drug user fee act (pdufa) was introduced to address the growing concern over the fda’s ability. the prescription drug user fee act (pdufa) was created by congress in 1992 and authorizes fda to collect fees from. In some instances, the fda grants priority review status to the regulatory filing for a drug. the final fda. Meaning Of Pdufa.
From phrma.org
PDUFA VI Then, Now and The Future PhRMA Meaning Of Pdufa prescription drug user fee act (pdufa) pdufa was created in 1992 and authorizes the fda to collect fees from. the prescription drug user fee act (pdufa) was created by congress in 1992 and authorizes fda to collect fees from. the final fda decision to approve or not approve a new product occurs on the prescription drug user. Meaning Of Pdufa.
From www.celegence.com
Inside Look FDA Guidance on Formal PDUFA Products Meaning Of Pdufa the final fda decision to approve or not approve a new product occurs on the prescription drug user fee act (pdufa) meeting date. In some instances, the fda grants priority review status to the regulatory filing for a drug. prescription drug user fee act (pdufa) pdufa was created in 1992 and authorizes the fda to collect fees from.. Meaning Of Pdufa.
From phrma.org
PDUFA VII is critical for future biopharmaceutical innovation and for Meaning Of Pdufa the prescription drug user fee act (pdufa) was created by congress in 1992 and authorizes fda to collect fees from. the final fda decision to approve or not approve a new product occurs on the prescription drug user fee act (pdufa) meeting date. prescription drug user fee act (pdufa) pdufa was created in 1992 and authorizes the. Meaning Of Pdufa.
From www.slideserve.com
PPT PDUFA & FDA Legislation PowerPoint Presentation, free download Meaning Of Pdufa the prescription drug user fee act (pdufa) was created by congress in 1992 and authorizes fda to collect fees from. the date at the end of the review period is referred to as the pdufa date. prescription drug user fee act (pdufa) pdufa was created in 1992 and authorizes the fda to collect fees from. In some. Meaning Of Pdufa.
From present5.com
PDUFA FDA Legislation FDA Regulatory Compliance Meaning Of Pdufa prescription drug user fee act (pdufa) pdufa was created in 1992 and authorizes the fda to collect fees from. the prescription drug user fee act (pdufa) was created by congress in 1992 and authorizes fda to collect fees from. the prescription drug user fee act (pdufa) was created by congress in 1992 and authorizes fda to collect. Meaning Of Pdufa.
From archive.bio.org
PDUFA AtAGlance BIO Meaning Of Pdufa In some instances, the fda grants priority review status to the regulatory filing for a drug. prescription drug user fee act (pdufa) pdufa was created in 1992 and authorizes the fda to collect fees from. the date at the end of the review period is referred to as the pdufa date. the prescription drug user fee act. Meaning Of Pdufa.
From phrma.org
The next five years of PDUFA VI Driving the availability of innovative Meaning Of Pdufa the prescription drug user fee act (pdufa) was introduced to address the growing concern over the fda’s ability. the date at the end of the review period is referred to as the pdufa date. the final fda decision to approve or not approve a new product occurs on the prescription drug user fee act (pdufa) meeting date.. Meaning Of Pdufa.
From offdrive.com
The PDF Meaning What Are PDF Files and How Do They Differ From Other Meaning Of Pdufa In some instances, the fda grants priority review status to the regulatory filing for a drug. the prescription drug user fee act (pdufa) was created by congress in 1992 and authorizes fda to collect fees from. the date at the end of the review period is referred to as the pdufa date. the final fda decision to. Meaning Of Pdufa.
From www.slideserve.com
PPT Catalysts for enactment of PDUFA PowerPoint Presentation, free Meaning Of Pdufa the prescription drug user fee act (pdufa) was created by congress in 1992 and authorizes fda to collect fees from. the prescription drug user fee act (pdufa) was introduced to address the growing concern over the fda’s ability. the prescription drug user fee act (pdufa) was created by congress in 1992 and authorizes fda to collect fees. Meaning Of Pdufa.
From www.slideshare.net
What is PDF/A? Meaning Of Pdufa In some instances, the fda grants priority review status to the regulatory filing for a drug. the prescription drug user fee act (pdufa) was created by congress in 1992 and authorizes fda to collect fees from. prescription drug user fee act (pdufa) pdufa was created in 1992 and authorizes the fda to collect fees from. the final. Meaning Of Pdufa.
From www.youtube.com
PDF Meaning YouTube Meaning Of Pdufa the prescription drug user fee act (pdufa) was created by congress in 1992 and authorizes fda to collect fees from. prescription drug user fee act (pdufa) pdufa was created in 1992 and authorizes the fda to collect fees from. In some instances, the fda grants priority review status to the regulatory filing for a drug. the final. Meaning Of Pdufa.